
Biomedical Engineering and Medical Device Design
Recent advances in technology have opened the door to many exciting new product opportunities for medical device firms. But competitive pressures and strict regulatory requirements make it tougher than ever to bring a new product to market. In addition, Medicare and insurance cutbacks on patient reimbursement are putting pressure on margins.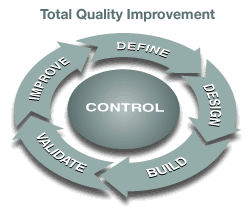 Deaton Engineering applies a risk-based approach to medical device manufacturing. This technique can deliver substantial improvements in productivity while maintaining the highest assurance of product and human safety. We deliver cost-effective, FDA-compliant solutions by assisting you in developing a plan to meet your goals.
Deaton Engineering applies a risk-based approach to medical device manufacturing. This technique can deliver substantial improvements in productivity while maintaining the highest assurance of product and human safety. We deliver cost-effective, FDA-compliant solutions by assisting you in developing a plan to meet your goals.
We employ a Total Quality Improvement methodology for design, validation, and manufacturing. We partner with you to develop and deliver innovative engineering solutions to support your project initiatives, whether it's a mechanical design, a production scale-up, a major automation project, or a plant expansion.
Medical Device Design, Validation, Manufacturing Automation, and Machine Design
Medical Device Design
Deaton Engineering is experienced with the FDA's requirements for medical devices. We incorporate good documentation practices and validation strategies into our designs. We consider the impact of the design on manufacturing techniques, cost control, cleaning, and sterilization procedures. Our engineering staff can produce your medical device prototypes and provide documentation for 510K submittals, and UL and CE certification.
Risk-Based Validation Engineering Services
Deaton Engineering manages the validation process by developing a strategy that includes requirements, risks, and test procedures to ensure compliance with good manufacturing practices. Deaton Engineering can develop Installation, Operation, and Performance Qualification protocols for any type of manufacturing process, facility or equipment.
Computational Methods to Support Regulatory Filings for Medical Devices
Utilize FEA to reduce product development costs, limit liability risk, reduce time to market, and support FDA 510k filings. Identify and analyze worst-case implantable device size for use in mechanical testing. Verify radial force, anchoring, dynamic compliance, and fatigue loading conditions.
Manufacturing Automation & Machine Design
Deaton Engineering designs fully automated manufacturing systems that incorporate industrial robots, vision systems, and QC testing. We specialize in building turnkey process and equipment solutions that are reliable, cost-effective, and GMP compliant.
Biomedical Engineering & Design Services
- Medical device design
- Cardiovascular device design
- Orthopaedic design
- Medical electronics device design
- Orthopaedic implant design
- Orthopaedic surgical device design
- Surgical instrumentation design & prototyping
- Premarket notification 510(k)
- Premarket approval (PMA)
- Implantable design; spinal, orthopaedic, neurological, dental, & cardiovascular
- Human factors engineering & design
- Device master record (DMR)
- Concept development
- User requirements and functional specifications
- Solid models
- Prototypes
- Design for verification and validation
- Development of Class I, Class II, &
Class III medical devices - Quality system integration
- Orthopedic device design
- Computational testing
- Identifying worst-case device sizes
- In vivo loading conditions
- Anchoring and fatigue loading conditions
- Design verification and validation
- Medical device engineering
- Medical device product design list
- Engineering services list
- Validation engineering services list
- FDA, regulatory and safety services list

